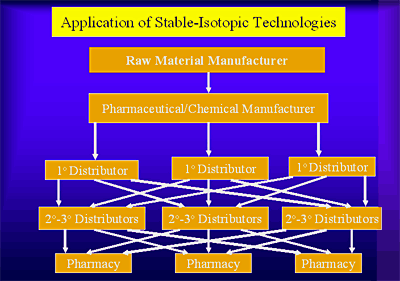
Pharmaceuticals are subject to various forms of identity fraud from the earliest stages of raw material acquisition though manufacturing and various steps in product distribution as illustrated here:
At any stage in acquisition, production, or distribution, pharmaceutical authenticity can be variously corrupted.
The General Structure of the Solution to Pharmaceutical Counterfeiting
The authenticity of pharmaceuticals is protected by an FDA - and pharmaceutical-industry tripartite program of security that includes overt, covert, and forensic technologies. Those technologies are summarized as follows (from Jasper, 2004).
- “Overt technologies are protective measures that are easily visible to the eye, such as holograms, color shifting dyes, and some watermarks.
- Covert technologies are protective measures that are not visible to the eye and frequently require special equipment for visualization (and authentication). These include some watermarks, certain inks and dyes that fluoresce or absorb ultraviolet light, and invisible bar codes.
- Forensic technologies are protective measures that require sophisticated analytical equipment analytical equipment, usually found in a forensic chemistry lab, in order to be identified. These include chemical markers, natural-stable isotopic tracers, or other unique chemical properties of a substance.